“let us hope that it is not true but, if it is true, let us make sure that it is not widely knownâ€
Anonymous comments to the idea of Evolution.
A pdf of this blog can be downloaded here.
Nature has dedicated two News and Views to a recent piece of work on Wingless (1), thus emphasizing its importance. Both comments focus more on the notion of Wingless as a morphogen than on other aspects of the work. The reason might lie in the fact that much of the notion about Wnt signalling in mammals is derived from the analysis and interpretations of work in Drosophila. The manuscript of Alexandre, Baena and Vincent (1) suggests that in Drosophila the long range action of Wingless is not functionally significant, thus inviting a reassesment of our understanding of the function of Wnt proteins. Surprisingly, both comments read like health warnings. Here I would like to comment on the one from Gary Struhl (2) who appears to cast some doubt on the ability of the experiments in the manuscript to debunk the belief that Wingless acts as a long range morphogen in Drosophila.
Gary Struhl has spent a life time extolling the virtues of Morphogens in pattern formation and devising experiments to prove their existence in Drosophila. His comment raises doubts over the findings of the paper but has a number of factual inaccuracies, misleading statements and missing references which need pointing out.
The opening statement of the News and Views makes clear how uncomfortable the author is with the work of Alexandre, Baena and Vincent: “There is compelling evidence that Wg can, and normally does, act over many cell diameters to control gene expression and growth of the Drosophila wing. So the remarkable discovery that a membrane-tethered form of Wg can substitute for the normal protein poses the question: must morphogens move to organize development? When considering this challenge to how we think of morphogens, the devil is in the detailsâ€. A strong statement to which a learnt reader can only say: really? where is that compelling evidence? A give away to its source is that the three references in support of the notion are two papers by Struhl himself (3, 4), and one by Cohen (5), another advocate of the Wingless = Morphogen notion. Self interest? Perhaps, as far as some of us remember, the best evidence was, as it appears to be now, the repetition of the mantra “Wingless acts long range and therefore it is a morphogen†which is in the title of many papers.
I agree with Struhl on one point: that the devil is in the detail. But perhaps not where he wants us to look into but rather in the work of the 90s. So, let us look at some details (with apologies to all who have heard the story before but Struhl’s views suggest that it is worth repeating and, in any case, there are a few new issues that I had not wanted to review earlier). I shall not discuss the work of Neumann and Cohen as it contains little to even discuss (in fact this manuscript is apt for that accolade of the physicist W. Pauli of ‘not being even wrong’) and, what is worth discussing, has been addressed in the literature (6).
The details the devil forgot
Let us start with what is missing.
It is far too often forgotten that there has been evidence in the direction of the work of Alexandre et al for over 20 years but that Struhl, Cohen and other prominent researchers, decided to ignore it throughout the 90s. The early arguments in favour of the Wingless = Morphogen notion were derived from work on the wing disc of Drosophila. Here the “idea†was that during larval development Notch signalling sets up a stripe of Wingless expression along the dorsoventral boundary of the developing wing disc which was thought to act as the Organizer/Morphogen/whatever-you-wanted-to-call-it, to rule the growth and patterning of the wing. This is, essentially, the view subscribed by Struhl. The number of papers and reviews supporting it during the 90s and 00s is very large to the point that the notion made it into textbooks and the psyche of developmental biology. In fact it has become the view in vertebrates where there is very little evidence to support it at the moment. But, as I have said before, there was phlogiston in the wing (http://amapress.gen.cam.ac.uk/?p=1191) and, particularly in the work of Struhl and others, the devil never looked at the details of the experiments and forgot others which challenged it.
In summary, there are two sets of experiments that were (and I may add are) consistently ignored that argued against their case. The first one, that removal of the stripe of Wingless did not affect the growth and patterning of the wing (7) (this has since been confirmed by the work of JP Vincent and his colleagues (8) which to their credit never fell for the Morphogen notion). This work is, once again, ignored in the commentary and should have been addressed as it is a sound observation that supports the findings of the paper (I should add that, for some reason, it is also not mentioned in 1). The second set of experiments involved attempts to rescue the loss of wing of Notch mutants with overexpression of Wingless or Armadillo –as the mantra says should happen. It never worked (6, 9). It has always been puzzling that people who talked about Wingless as a Morphogen never deigned to even discuss these results. Late in the game Giraldez and Cohen tried hard to exorcise them but could not (10); even Struhl himself had to accept some of them (11).
Warning: One experiment that was used by some people to claim an involvement of Wingless in the growth of the wing is the emergence of an ectopic wing from the notum (apologies for the technicality) when Wingless is overexpressed in a particular region of the wing disc. The interpretation of the experiment is a misconception and a misunderstanding of the development of the disc. It would take too long to discuss this and I refer the interested reader to places where an explanation has been elaborated at length (6, 9, 12). In essence, the pattern of expression hits a sweet spot for the initiation of wing development and what one is doing is using Wingless to start (not to organize) the development of another wing.
Another detail against the Wingless=Morphogen notion concerns the effects that Wingless has on proliferation, or growth if you will. This is a subtle issue but, attempts to look for an effect of Wingless signalling on proliferation in the wing have been unsuccessful (NB in the sense of whether loss or gain of Wingless or ß-catenin alone affects proliferation e.g 13). However, loss of function of the receptor, Arrow (LRP5/6), does affect viability (10) and there are some truly intriguing observations from mosaics in experiments of the Vincent lab (14 and see also 8) that deserve some close attention. They suggest that the interactions of Wingless signalling with other pathways might be more important than what Wingless can do on its own (see e.g 15). This is most clearly seen in its interactions with Vestigial (9 and also discussed very nicely by Struhl in 4). But this is a different matter and does not detract from one fact: there is little evidence that, on its own, Wingless affects growth, and certainly none that it does so as a long range signalling molecule.
In summary: there is abundant evidence that the stripe of Wingless expression that emerges at the same time in the first day of the third larval instar does not act as a long range instructive source of Wingless for the development of the wing.
The devil in the roots of the mantra: the “direct and long range action of Winglessâ€
Here I would like to address a number of issues on the results of the work from Struhl’s lab which is the cornerstone of his views of Wingless as a Morphogen (3). This might be tedious for some readers so I shall summarize; for those interested in details (and there are some juicy ones) I suggest you go the Appendix or/and download a full length version of this post at the top.
In summary, a close look at the results in this work (3 published in Cell) reveals
1. Low quality data
2. Uncritical analysis of the results which, for the most part, are made up of a series of selected correlations
3. No details of how the crucial experiments were performed (no genotypes, no details of developmental timings, no landmarks that allow one to evaluate the experiments)
4. No epistasis which would allow a test of whether Wingless is or is not a Morphogen
5. Most worryingly, an experiment in Figure 1 which is either impossible or, at best, puzzling. Having consulted with colleagues familiar with the system none of them understands what panels E and F are and, if they are what is said, how are the results obtained (for details see Appendix).
Unfortunately, details are not a strength in the papers quoted by Struhl, particularly about timings and patterns of expression of Wingless. This perhaps explains the lack of understanding of the work of Alexandre et al. as Struhl and colleagues have rarely strayed from the third instar discs. As clearly shown and discussed by Alexandre et al (and see also references 6, 7, 9, 12, 16. 17 and timeline) the DV stripe of Wingless emerges rather late, certainly not before the beginning of the third larval instar, by the time the wing has almost finished the bulk of its growth. Before this, Wingless goes through a succession of patterns but not in the manner relayed by Struhl : “On the first day, Wg, is broadly expressed, but its expression fades progressively in the more dorsally and ventrally positioned cells, generating Wg gradientsâ€. This is an oversimplification of a sequence of events that take place over four days and involve not the simple change minimalistically depicted in the News and Views but a series of patterns of patterns of expression under different controls (see Figure below and 12, 16, 17). A failure to grasp the details of this sequence of events and take them into account in the design and interpretation of experiments can be fatal. And in the case of reference 3, it is (see Appendix)
Another feature of the News and Views is the clear mistake in the accompanying figure. I am sure that Struhl has nothing to do with this but notice that the Nrt-Wingless (membrane tethered) wing of the figure shows a reduction of about 35-40% of the normal size, whereas the same wing in the manuscript (the same wing! The same experiment!) shows a much smaller reduction of about 10%. This means that the effect is exaggerated in the News and Views in a manner that suggests, falsely, that the Nrt-Wingless wings are not normal –which is not the conclusion of Alexandre et al.  Given the interest of the News and Views to question the results of Alexandre et al. if Struhl has not had access to the flies, I would suggest that this is a manipulation of the original data to favour the point of the commentary. The legend of this figure ends up with a statement: it remains unclear how the long-distance Wg signalling thought to be required for wing development is exerted in these flies. Well, leaving aside the manipulation of the figure, perhaps the answer lies in that THERE IS NO LONG RANGE FUNCTION OF WINGLESS.
Figure shows wings from the wild type (outer outline) and Nrt-Wingless flies as reported in the manuscript of Alexandre et al (red dotted rim) and as represented in the News and Views by Struhl (blue dotter inner rim). Notice that the inner perimeter is said to represent the red dotted one.
In summary: the dangers of being seduced by the ideas
The problem with looking at details is that, in the case of Wingless=Morphogen, the more one digs into the experimental foundation of the idea, the harder it is to understand not just the claims of the News and Views but how the developmental biology community was seduced by an idea for which there was so little evidence. Struhl appeals to the need to look at the small print in the experiments of Alexandre et al. and in doing so is grasping at straws. Is he claiming that homeopathic quantities of Wingless liberated from the membrane tether are mediating the morphogen function? I suggest that he looks into his experiments and sees how much of the evidence he uses can be sustained today. His arguments read like the comments of a polite reviewer 3 whose experiments have not yet been done. More to the point they echo that victorian lady commenting on Darwinism: let us hope that it is not true but, if it is true, let us make sure that it is not widely known.
References
1. Alexandre, C., Baena, LA. Vincent JP. (2014) Patterning and growth control by membrane-tethered Wingless. Nature 505, 180-185.
2. Struhl, G. (2014) Long range thinking. Nature 505, 162-163.
3. Zecca, M., Basler, K. & Struhl, G. Cell 87, 833–844 (1996). Direct and long-range action of a wingless morphogen gradient.
4. Zecca, M. & Struhl, G. (2007) Development 134, 3001-3010. Control of Drosophila wing growth by the vestigial quadrant enhancer.
5. Neumann CJ, Cohen SM. Long-range action of Wingless organizes the dorsal-ventral axis of the Drosophila wing. Development. 1997 Feb;124(4):871-80.
6. Klein, T. Martinez Arias, A. (1998). Different spatial and temporal interactions between Notch, wingless and vestigial specify proximal and distal pattern elements of the wing in Drosophila . Dev. Biol. 194, 196-212.
7. Couso, J.P., Bishop, S. Martinez Arias, A. (1994). The wingless signalling pathway and the patterning of the wing margin. Development. 120, 621-636.
8. Piddini E, Vincent JP. (2009) Interpretation of the wingless gradient requires signaling- induced self-inhibition. Cell 136, 296-307.
9. Klein, T. Martinez Arias, A. (1999). The Vestigial gene product provides a molecular context for the interpretation of signals during the development of the wing in Drosophila. Development 126, 913-925
10. Giraldez AJ, Cohen SM. (2003) Wingless and Notch signaling provide cell survival cues and control cell proliferation during wing development. Development 130, :6533-6543.
11. Zecca M, Struhl G. (2007) Control of Drosophila wing growth by the vestigial quadrant enhancer. Development 134, 3011-3020.
12. Martinez Arias, A. and Stewart, A. (2003) In molecular principles of development. OUP. Look up at  Fig 12.21 and the associated section pp 382-389.
13. Johnston, L. Sanders, AL. (2003) Wingless promotes survival and constrains growth during Drosophila wing development. Nature Cell Biol. 5, 827-833.
14. Baena-Lopez LA, Franch-Marro X, Vincent JP. (2009) Wingless promotes proliferative growth in a gradient-independent manner. Sci Signal. 2009 Oct 6;2(91):ra60. doi: 10.1126/scisignal.2000360.
15. Herranz, H., Perez, L., Martin, F.A. and Milan, M. (2008) A wingless-Notch double repression mechanism regulates G1-S transition in the Drosophila wing. EMBO J. 27, 1633-1645.
16. Martinez Arias, A. (2003) Wnts as morphogens? The view from the wing of Drosophila. Nature reviews in Molecular Cell Biology 4, 321-325.
17. Hayward, P., Kalmar, T. Martinez Arias, A. (2008) Wnt/Notch signalling and information processing during development Development 135, 411-424.
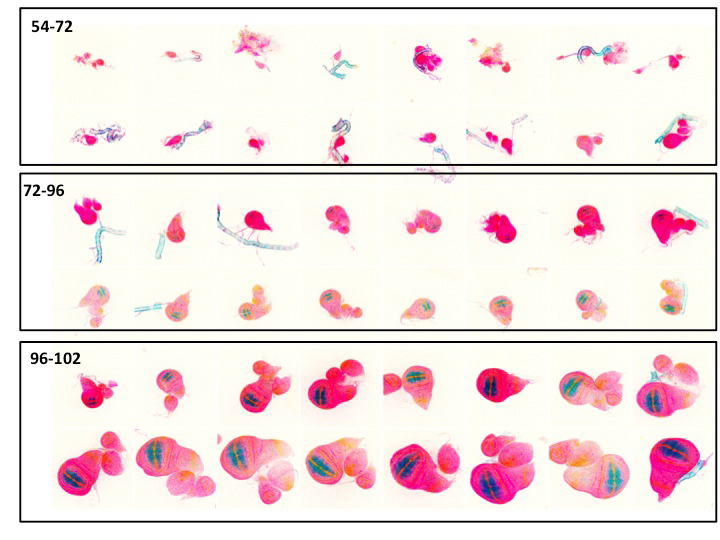
Collection of batches of wing imaginal discs of the indicated ages, determined as hours from egg laying. The blue staining represents the vgQE; compare to Fig 1. As the second instar (L2) happens between 72 and 96 hrs AEL, one can see here that much of the growth of the wing happens at the boundary of the second and third larval instars and that, after half way through the first part of L3, the wing does not grow vert much. The QE, which is a faithful reporter of the wing, only starts at the end middle and the end of L2 (Cassie Yu Bian and AMA)
Acknowledgements: I want to thank three colleagues who have helped me craft this discussion. JP Couso, T. Klein and M. Milan have been helpful and critical on this matter. They have also made contributions to the field and, notably, to the issues raised by this work and both News and Views. It is unfortunate that their work has been forgotten, as it does enlighten and broaden the subject in addition to provide support for the conclusions of Alexandre et al.
Appendix
On details of mutants, details of timings, details of patterns, details of appendages, details……
These are some comments on the work of Zecca, M., Basler, K. & Struhl, G. Cell 87, 833–844 (1996). Direct and long-range action of a wingless morphogen gradient.
The papers quoted by Struhl (A1, A2) in support of the notion that Wingless acts as a long range morphogen, claim to show that diffusible rather than membrane tethered Wingless could change the levels of expression of presumed targets like Distalless and Vestigial. Two coments on this.
First of all, nobody disputes that Wingless can diffuse and does diffuse during development. The point is whether this diffusion has functional significance. In the experiments  in ref. A1 there are no functional tests so, one cannot conclude much of substance to the arguments from the reported observations. It could be argued that it was shown that Wingless can change the levels of Distalless and Vestigial. Probably, but the observations in this report (I mean what is shown) are questionable as there are few and selected examples. The bulk of the data for this is in Figures 3 and 4. It could be the techniques of the time and the lack of details and controls, but there are many questions on these figures, particularly if they are the best examples in support of the long range actions of Wingless.
In Figure 3, the lasers of the confocal are so high that all constructs appear to have a long range effect. It would have been good to have some controls and cellular resolution, particularly as it is distance from source that is at stake here. Notice, for example, that in 3F there is a lot of ectopic Dll in places where there is no expression of Armadillo (incidentally, it is very difficult to work out what the authors are looking at; not only there is no orientation of the discs –what is anterior or posterior- nor staging, there is no wild type reference for these experiments. Furthermore, the authors do not use the endogenous Dll expression but a Dll-LacZ which does not recapitulate the endogenous expression and appears to be somewhat sensitive to Wingless signalling –unpub. obs-).
Also, to highlight but another example, it is odd that in 3D the ‘long range’ effect of Wingless on Vg is only on one side of the disc (the left) and not on the other (the right). These examples, crucial to the argument, suffice to show that they are not only insufficient but inappropriate for the case. An effect of Wingless on the levels of expression of Vestigial has been shown so, they are right, but the effects of Dll (not the enhancer used here) are more difficult to gauge and this is crucial for the notion of Morphogens.
Figure 4 raises many questions. A look at it shows that the ‘induction’ of neur-Z is very sparse. It is surprising how few cells are led to express it in A, there is very little non-autonomy in C and in E the questions arises why is it that only few cells in the clone express it. Needless to say that once again, as in Fig 3, we do not know what are we looking at: which part of the disc, which stage, etc. This problem with details were at the time, and can be seen now, one of the major issues with this work.
But in the end, accepting that Wingless signalling alters the levels of expression of Vestigial and Distalless, these changes in expression appear to be functionally irrelevant. Although no functional test exists in ref. A1. JP Vincent and his colleagues have shown that removal of the DV stripe of Wingless from the developing disc, does not affect the patterns of Distalles and Vestigial (A3), which fits the earlier observations of the lack of an effect of the stripe of Wingless on the patterning of the wing (A4). There are also other examples which show that Wingless is not necessary for the growth of the wing which should have been discussed. Just to quote one: apterous mutants lack a wing but when they are combined with a mutant for Hairless (a negative regulator of Notch signaling) a wing grows without a margin and without Wingless expression (A5). This phenotype is similar to the one that results from removing the DV stripe of Wingless and reinforces the notion that the DV stripe of Wingless does not act as a source of long range morphogen required for the growth and patterning of the wing but rather as a local signal for neurogenesis at the anterior margin.
The wing is not the only place where Wingless has been made into a Morphogen and some of the early work of Struhl on Wingless deals with the leg (A6). Much less attention has been paid to the patterning of the leg discs, at least in the public domain, but detailed experiments carried on after the work on the wing have not found evidence for a long range patterning activity of Wingless (A7-A10). By now it is well established that in Drosophila, the pattern of the leg is established though a sequence of mutually dependent interactions between gene regulatory networks in a background of Wingless activity. The similarity with the wing disc being uncanny and the relevance of these observations to the findings of Alexandre et al. obvious. Once again, the silencing of these results and their relevance to earlier statements is, at the very least, surprising and reveals Occam’s broom at work.
A most important detail. The work of Zecca et al (A1) has one loss of function experiment in support of the long range action of Wingless in the growth and patterning of the wing. The experiment is shown in Figure 1, particularly panels E and F. The legend reads:
“(E and F) Dll (E) and vg (F) expression in wgts wing discs 2 days after a shift to the non permissive temperature: Dll expression in the wing is abolished, and vg expression is reduced to a thin stripe of cells along the DV compartment boundaryâ€
The devil might be seriously in the detail here. It is difficult to know when the temperature was shifted as we are not told the age of the discs and this, given the changing patterns of Wingless expression, is crucial to interpret the observations. The experiment is not easy (though there is no description of how it is done so it is difficult to reproduce) since growth at the permissive temperature (18oC) is slower than at 25oC which adds to the difficulty of interpreting the outcome of the experiment. Notwithstanding this, the figure contains some landmarks that allow us some useful inferences.
The leg disc on the left in panel E suggests that the shift must have happened late since the expression of Dll in this disc depends on Wingless early (the authors know this). If the shift had been done early, Dll would have disappeared from both. As it is expressed in the leg, the shift must have taken place at least 48 hours AEL (at 25oC). It should also be pointed out that this disc has a sizeable pouch (gauged from the rings that define the hinge), smaller than wild type but large, which suggest that the shift has been made so late (in the 25oC timeline) that there is little effect on the growth of the wing (although a proper analysis of this depends on timings and stagings that we are not given): as we do not know the age of the disc nor how much time it has been at 25oC (or is it 29oC?) it could well be that the reason for the small size of the wing pouch has to do with the young age of the disc. However, notice that despite this the authors want us to see here lack of growth of the wing. The pattern of Vestigial in F tells us a few more things about this experiment. The pattern shown is surprising, as in the experience of those who have done these experiments, in the absence of Wingless, Vestigial is either gone or present but never maintained in a DV stripe which, by the way, resembles the vestigial Boundary Enhancer (BE) that was first characterized by S Carroll’s lab. So, it could be that for some reason they have uncovered an endogenous pattern that is dependent on the BE which, incidentally, is independent of Wg once it has been activated. Whatever it is and wherever it comes from, this pattern of expression provides another hint of when the shift was done as it suggests that the DV boundary had been formed at the time of the shift (Wingless is necessary for the DV boundary to form properly by regulating the expression of Delta (reviewed in A11). As in E, this places the shift very late since the DV compartment boundary is not established before 48 hours. In this panel, as in E, there is wing pouch and with all the caveats raised above and the lack of detail of how the experiment was done, this would suggest that the shift was done about 72 hrs+  (in the 25oC timeline) and that removal of Wingless at this time, whether it has an effect on Dll and Vestigial or not, it has little effect on the development of the wing, as has been shown before and later (A3, A4).
One added small detail. There is something difficult to understand here, and when one looks for details in the materials and methods, there are none. More significantly, some of us would like to know which wg ts (IL114 allele one presumes) chromosome did the authors use. The reason to ask is because if it is the original one from the Nusslein-Volhard collection, it happens to have an associated lethal which makes this type of experiments impossible –tried and tested in reference 7. In fact this is the reason why in our work (A4) we used different chromosomes and often in trans. As there are no details of how this experiment was done, what chromosomes were used, how the mutants were recognized and what was the timing of the shifts, it is very difficult to understand what is going on. Some details would have been and still would be welcome.
Corollary
There might be a more fundamental and interesting reason for Struhl’s problems with these results. In particular one that, if true, highlights the limitations of a strict genetic analysis of development. Struhl is, first and foremost, a geneticist; probably one of the best developmental geneticists of the 80s and the 90s. He uses genetics to infer mechanisms following a straightforward method based on the analysis of mutant phenotypes: 1) set up a genetic situation, often clones of gain or loss of function of a particular gene; 2) let the system run its course; 3) obtain a result, usually in the form of a terminal or quasi terminal phenotype, and 4) use the outcome of the experiment, together with a hypothesis, to infer what has happened i.e. what kind of mechanism might lie underneath the observed result. The essence of this method is that it works with a black box which contains the mechanism. The experiments of Alexandre et al. have a similar flavour but have many elements of cell biology and show an interest in understanding, rather than inferring, what is in the black box. Struhl has decided what there is in the black box and therefore, in his view, any result against it (what he believes to be there, simply because it is his favoured explanation) needs to be looked at carefully. He should realize that today we have methods that can crack the black box. Genetics is a beautiful formal intellectual construct which has provided undoubted insights into many processes but it has done best when going hand in hand with other complementary techniques like cell biology or biochemistry. The work of Alexandre, Baena and Vincent does this and, in doing so, advances our understanding of Wingless signaling in development.
So, I would suggest that part of the problem of Struhl has to do with his repeated attempts to use classical and limited techniques and frameworks against more rigorous and modern approaches to the problem.
Appendix References
A1. Zecca, M., Basler, K. & Struhl, G. Cell 87, 833–844 (1996). Direct and long-range action of a wingless morphogen gradient.
A2. Zecca, M. & Struhl, G. (2007) Development 134, 3001-3010. Control of Drosophila wing growth by the vestigial quadrant enhancer.
A3. Piddini E, Vincent JP. (2009) Interpretation of the wingless gradient requires signaling- induced self-inhibition. Cell 136, 296-307.
A4. Couso, J.P., Bishop, S. Martinez Arias, A. (1994). The wingless signalling pathway and the patterning of the wing margin. Development. 120, 621-636.
A5. Klein,T., Seugnet, L., Haenlin, M. and Martinez-Arias, A. (2000).Two different activities of Suppressor of Hairless during wing development in Drosophila. Development 127, 3553-3579
A6. Struhl, G. and Basler, K. (1993) Organizing activity of wingless in Drosophila. Cell 368, 527-540.
A7. Galindo, MI., Bishop, S., Greig, S. and Couso, JP. (2002) Leg patterning driven by proximal-distal interactions and EGFR signaling. Science 297, 258-259.
A8. Campbell, G. (2002) Distalization of the Drosophila leg by graded EGF-receptor activity. Nature 418, 781-785.
A9. Estella C, Voutev R, Mann RS. A dynamic network of morphogens and transcription factors patterns the fly leg. Curr Top Dev Biol. 2012;98:173-98.
A11. Kojima, T. (2004) The mechanism of Drosophila leg along the proximo distal axis. Dev. Growth Diff. 46, 115-129.
A11. Hayward, P., Kalmar, T. Martinez Arias, A. (2008) Wnt/Notch signalling and information processing during development Development 135, 411-424.