COI: our group has an interest in these matters and the post reflects this and contains references to our work.
COI: our group has an interest in these matters and the post reflects this and contains references to our work.
The idea of making a human being from natural or unnatural parts has been more than a curiosity for centuries. The story is told in a little known book by Philip Ball (“Unnatural: the heretic act of making people†Vintage books, London 2012) and has many a fascinating angle, though the bit I really enjoy is the development of in vitro fertilization by Patrick Steptoe and Robert Edwards. So much Biology behind what today is a routine clinical procedure! This is an important point: whatever technology there is around, it is underpinned by basic Science. No application can be developed without the support and inspiration of a scientific question and this applies to making cars or humans. But, why would one want to make a human being in a lab?
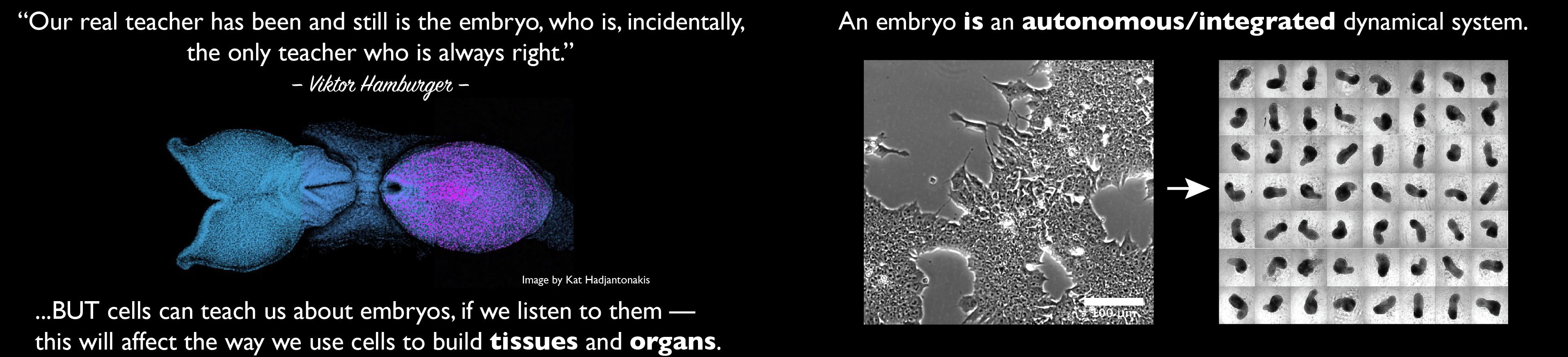
If you want to make a human all you have to do is to combine an egg with sperm and let it run. The entry of the sperm into the egg triggers a process that generates an embryo which, in turn, lays down the templates for tissues and organs and their relative organization in space. Leaving aside parthenogenesis (the activation of the process without fertilization), this is the way you make any animal and, in many ways, this is what Steptoe and Edwards did for the human by recreating the event in laboratory conditions. However, the process of activating development does not tell you much more than what you knew already since, once the process is triggered you are back to just watch how form unfolds over time i.e. Life, if you want to be dramatic, requires Life and all its pieces working together harmoniously. Can we do better? The advent of Embryonic Stem Cells (ESCs) has given the age old challenge of creating organisms in the laboratory a new lease of life, though very few people would look at it from the perspective of the actual making of a human.
ESCs are clonal derivatives from mammalian blastocysts that can be grown in culture and differentiated in a controlled manner into most cell types of an organism. Recently it has been found that these cells have the ability to organize themselves into tissues and organs and that we have the capacity to steer (but not to control) these processes. Thus disembodied brains, eye cups, intestines and muscles emerge from culture dishes under so called ‘defined conditions’. These structures are imperfect, often functionally wanting and, for the most part, just happen (rather than are created) and promise much more than they can deliver at the moment. Importantly, amplified by the media, they create dangerous hype and false hope. Intriguingly, they also raise questions about what is an organism that we have not thought much about yet.
A different approach to the problem is to go beyond the organs themselves and ask the question of whether ESCs can be used to build an organism or, for starters, an embryo. There are two reasons for this. Here I shall only state them and hope to find some time to expand on them in the near future. The first one is the sheer scientific value of what one can learn from such an experiment about how organisms are made. The second one, probably more practical, suggests that if you want to build tissues and organs, you better try to copy the embryo, to imitate Nature. Perhaps by starting with embryos one can endow cells with the properties of the real objects, improve the yield of organs and tissues and get them to a functional state. An interesting consideration arises from these musings: is it not what Steptoe and Edwards did an imitation of Nature? Did they not get an embryo out of a dish? Sure, in the end the embryo, the mammalian embryo, will need the mother but they did create a human embryo in the lab. But what do we learn from such imitations about the emergence of form? If one wants to learn how cells make embryos and we rely on the properties of the system to create embryos, what have we learnt? By counterfeiting a painting you don’t learn about the process that created it. Science teaches us something from decomposing, from asking about minimal conditions. A good example can be found in the roots of Developmental Biology (the branch of Biology that studies the emergence of an organism from a fertilized egg). For many decades in the XIX century biologists looked at and described the embryonic development of different organisms and thus developed a good roadmap of what happens after fertilization. It was the agenda laid down by Wilhem Roux and its dramatic execution by Hans Driesch with his embryo splitting experiments, that teaches us something, namely the regulative power of embryos and, in doing so, it lays down the agenda for Experimental Embryology and later Developmental Biology in the XX century ( see A New Sort of Engineering I and II).
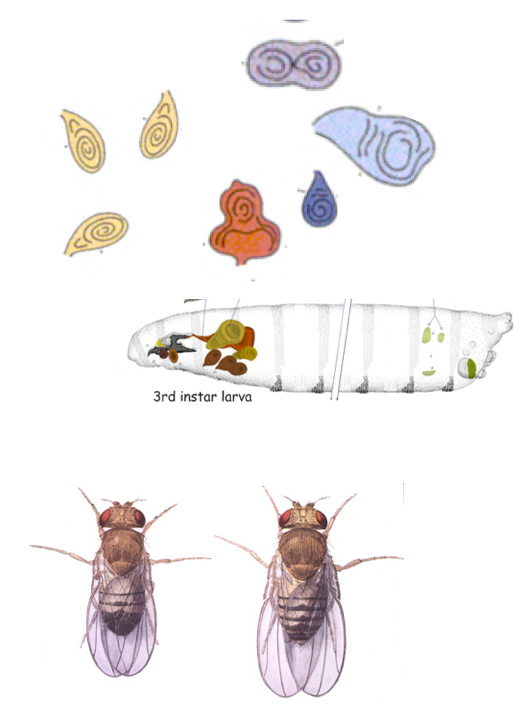 The initial question behind the experiments of Driesch and what followed was to find out the minimum number of elements that can give rise to an organism but, from there and because of the answers that emerged, this approach has been rich in contributions to our understanding of biological systems. The reductionist/deconstructionist approach that underlies Experimental Embryology generated a very successful research program that was further underpinned by Genetics: instead of using scalpels and needles, use mutations to understand the system. Genetics has shown not only the processes but has identified the parts. Its combination with molecular biology has proven most effective in the dissection of the entrails of the System and has revealed that the making up of an organism is not what it seems. A classic favourite of mine is the organization of the early Drosophila embryo into stripes, beautifully choreographed in their emergence and spatial organization but which experimentation shows are individually controlled elements of an evolutionarily fitted system. The visible coordination, the obvious symmetry is an illusion created by Natural Selection; the system is built piecemeal with the whole being a collection of parts, seamlessly woven into each other for, probably, function, over millions of years of tinkering. This should not come as a surprise. After all, how are Diptera (flies) made but from disconnected parts, called imaginal discs, which develop autonomously and independently from each other inside the larva (a feeding machine that provides the raw energy and matter for the growth of the parts of the adult). Although the imaginal discs do not raise eyebrows, particularly for those that work with them, it is a strange thing that legs, eyes, genitalia and wings develop independently from each other only to come together in an assembly line during metamorphosis in the ultimate example of self-assembly.
The initial question behind the experiments of Driesch and what followed was to find out the minimum number of elements that can give rise to an organism but, from there and because of the answers that emerged, this approach has been rich in contributions to our understanding of biological systems. The reductionist/deconstructionist approach that underlies Experimental Embryology generated a very successful research program that was further underpinned by Genetics: instead of using scalpels and needles, use mutations to understand the system. Genetics has shown not only the processes but has identified the parts. Its combination with molecular biology has proven most effective in the dissection of the entrails of the System and has revealed that the making up of an organism is not what it seems. A classic favourite of mine is the organization of the early Drosophila embryo into stripes, beautifully choreographed in their emergence and spatial organization but which experimentation shows are individually controlled elements of an evolutionarily fitted system. The visible coordination, the obvious symmetry is an illusion created by Natural Selection; the system is built piecemeal with the whole being a collection of parts, seamlessly woven into each other for, probably, function, over millions of years of tinkering. This should not come as a surprise. After all, how are Diptera (flies) made but from disconnected parts, called imaginal discs, which develop autonomously and independently from each other inside the larva (a feeding machine that provides the raw energy and matter for the growth of the parts of the adult). Although the imaginal discs do not raise eyebrows, particularly for those that work with them, it is a strange thing that legs, eyes, genitalia and wings develop independently from each other only to come together in an assembly line during metamorphosis in the ultimate example of self-assembly.
Drosophila has taught us much but there is one feature which is very revealing and is always overlooked as trivial, namely this coming together of the adult organism from parts, that the whole that we see flying around is an illusion created by the convergence of chance and necessity, of teleology and Natural Selection working together for reasons and benefits that we do not understand.  What the development of Drosophila tells us is that Biology is not intuitive and that behind a seamless whole there is much patchwork. But what does this have to do with making humans? Allow me to rephrase the question, rather than making humans -which I would argue has already been achieved- what we want is ways to learn how humans make themselves, how cells make humans. At this, perhaps we should aim lower first and start with a system we can work with and which is sufficiently similar to teach us the ropes. How about mice and, at even at a humbler level, mouse embryos? If we can make the seed, for that is what an embryo is, maybe we can learn about the organism. As usual, there are many ways to ask questions and we and others are trying 1 but while some aim to counterfeit the embryo, some of us have decided to see what cells can do if we set them free or if we constrain them. For example, arranging ESCs in constrained spatial arrangements and asking them to differentiate is teaching us about the connection of specific genetic circuits and the spatial self-organization of differentiating ESCs 2, 3. 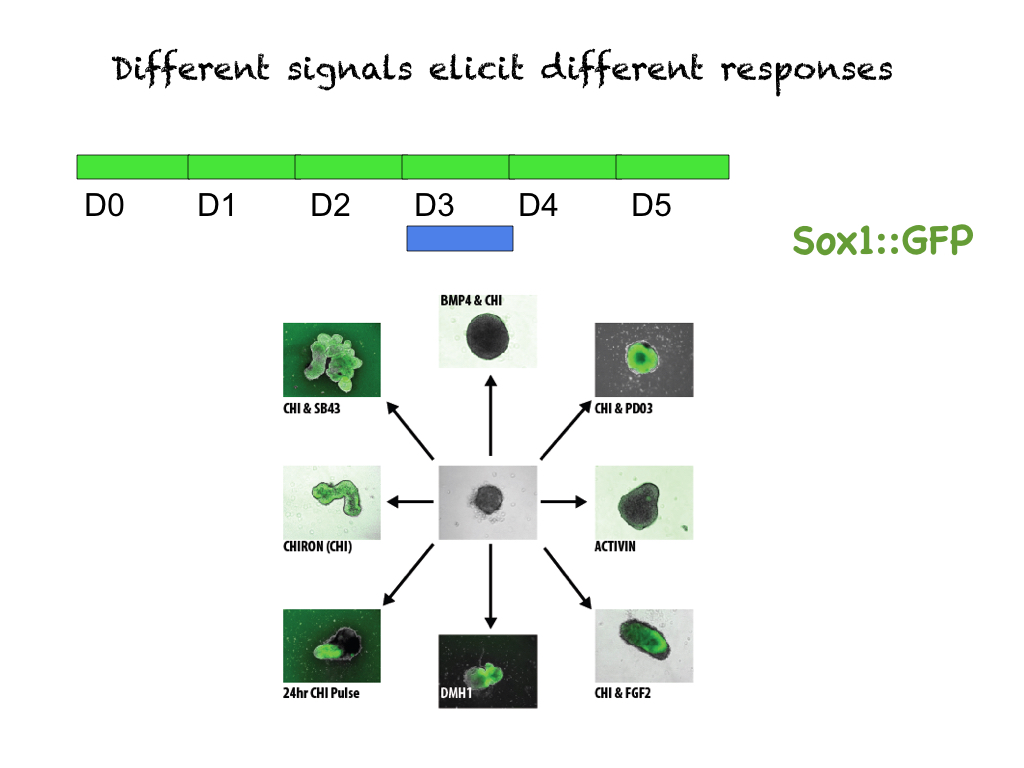 On the other hand, the development of 3D gastruloids has revealed many surprises as it looks as if, against the established wisdom of the field, the development of the axes of a mammalian embryo does not require the extraembryonic tissues 4-6. Furthermore, this work (still in its early days) reveals what could be interpreted as the equivalent of the imaginal discs of the mouse: that the head and the trunk can emerge independently of each other and that depending on the signals the cells receive at a particular time, they will assemble into specific and different parts of the embryo 7. I can hear the embryologists raising their voices and claiming that this is an artefact of the experimental system and this echoes much of what was in the air when Drosophila started to reveal the modular nature of its inner engineering. It may be, but I suspect that they are telling us something and that these contraptions are real.
On the other hand, the development of 3D gastruloids has revealed many surprises as it looks as if, against the established wisdom of the field, the development of the axes of a mammalian embryo does not require the extraembryonic tissues 4-6. Furthermore, this work (still in its early days) reveals what could be interpreted as the equivalent of the imaginal discs of the mouse: that the head and the trunk can emerge independently of each other and that depending on the signals the cells receive at a particular time, they will assemble into specific and different parts of the embryo 7. I can hear the embryologists raising their voices and claiming that this is an artefact of the experimental system and this echoes much of what was in the air when Drosophila started to reveal the modular nature of its inner engineering. It may be, but I suspect that they are telling us something and that these contraptions are real.
What this says is that we are moving into an era in Developmental Biology in which Engineering is going to play a central role and will do so in two ways. One, in the manner that we usually understand engineering i.e. the development of efficient and reproducible ways towards a practical end. This has already had an impact in Biology and Biomedical research: genetic engineering, as well as artificial hearts, bones and limbs bear witness to this. Now we are starting to apply this to the activities of ESCs, though we should be careful to control the hype that can surround this research. In addition, we should look at engineering as a discovery tool. In some ways and inadvertently, we have already since when Driesch and his followers start chopping the embryo into pieces, they are using some engineering principles. They are doing it in a crude way (can a biologist fix a radio?) but they are. The analysis of the stripes in the early Drosophila embryo is another more sophisticated example of this and one that has revealed some profound principles of the link between the molecular logic of a cell and how it is used to build the blueprint of an organism.
I do not see a reason to build an embryo of a mouse or of a human. This is a relatively interesting headline-grabbing challenge. Â On the other hand, I do see many reasons to ask questions about how cells build organisms or how much of an organism can arise from the autonomous behaviour of cells. In doing so we are learning interesting facts. For example that there are length scales in the organization of the basic cell populations (germ layers) that make up an embryo and, significantly, as pointed out above that mammalian embryos are, like Drosophila, piecemeal entities put together by evolution. In some ways, the emergence of organs in a dish hints at this but the reliability and reproducibility of this event might need the generation of the components of an embryo. I suspect that in understanding this there are prizes that will help us in the long term goal of engineering tissues and organs. Rather than imitating Nature we should aim to break the mirage that Nature and our minds have created in this wonderful balanced and continuous unit that we call an organism.
Note. The figure at the top is “the human condition” from R. Magritte.
- Simunovic, M. & Brivanlou, A.H. Embryoids, organoids and gastruloids: new approaches to understanding embryogenesis. Development 144, 976-985 (2017).
- Warmflash, A., Sorre, B., Etoc, F., Siggia, E.D. & Brivanlou, A.H. A method to recapitulate early embryonic spatial patterning in human embryonic stem cells. Nat Methods 11, 847-854 (2014).
- Etoc, F., Metzger, J., Ruzo, A., Kirst, C., Yoney, A., Ozair, M.Z., Brivanlou, A.H. & Siggia, E.D. A Balance between Secreted Inhibitors and Edge Sensing Controls Gastruloid Self-Organization. Dev Cell 39, 302-315 (2016).
- Turner, D., Glodowski, C., Alonso-Crisostomo, L., Baillie-Johnson, P., Hayward, P., Collignon, J., Gustavsen, M.W., Serup, P., Schroter, C. & Martinez Arias, A. Interactions between Nodal and Wnt signalling Drive Robust Symmetry-Breaking and Axial Organisation in Gastruloids (Embryonic Organoids). bioRxiv doi.org/10.1101/051722. (2016).
- Turner, D., Alonso-Crisostomo, L., Girgin, M., Baillie-Johnson, P., Glodowski, C., Hayward, P., Collignon, J., Gustavsen, M.W., Serup, P., Steventon, B., Lutolf, M. & Martinez Arias, A. Gastruloids develop the three body axes in the absence of extraembryonic tissues and spatially localised signalling. bioRxiv doi.org/10.1101/104539 (2017).
- van den Brink, S.C., Baillie-Johnson, P., Balayo, T., Hadjantonakis, A.K., Nowotschin, S., Turner, D.A. & Martinez Arias, A. Symmetry breaking, germ layer specification and axial organisation in aggregates of mouse embryonic stem cells. Development 141, 4231-4242 (2014).
- Baillie-Johnson, P., van den Brink, S., Balayo, T., Turner, D.A. & Martinez Arias, A. Generation of aggregates of mouse ES cells that show symmetry breaking, polarisation and emergent collective behaviour. JOVE doi: 10.3791/53252 (2014).