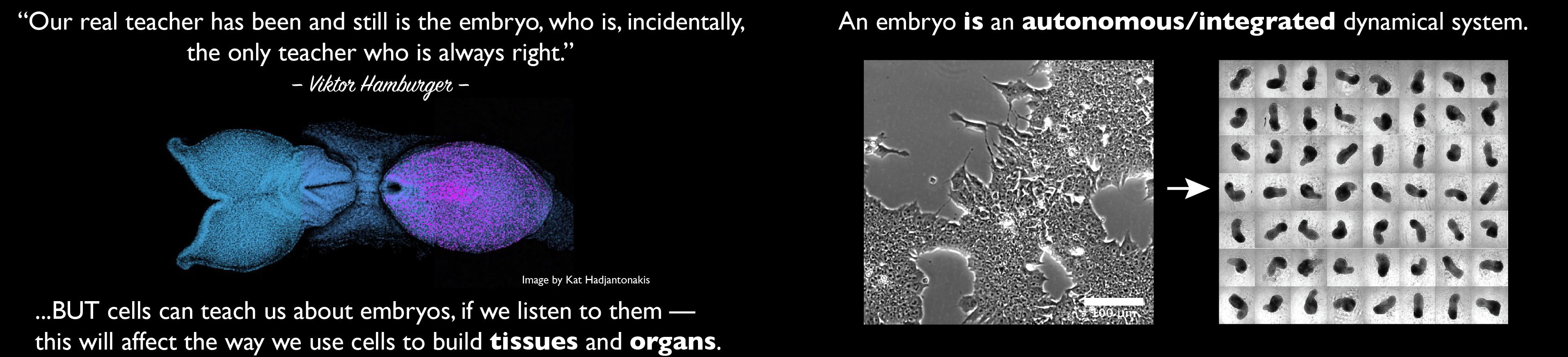
II. The 14-day rule and its implications
The 14-day rule is an important landmark in the study of the development of human embryos. Established as part of the conclusions of the Warnock committee of Inquiry into Human Fertilisation and Embryology (1), later enshrined into British law by an act of Parliament in 1990, it demarcates a line that cannot be crossed when growing human embryos ex vivo and has served as the reference for work with human embryos since. At the time, 1984, the ability to culture fertilized human eggs ex vivo did not go beyond three or four days, after which time they needed to be implanted to survive. However, the success in viability of the in vitro fertilized ova that was observed after implantation, raised the possibility that in the future, the creation of artificial conditions that mimicked implantation would allow this time to be extended and, even, that at some point embryos with full human potential might be grown in a dish. With this in mind, the Warnock report stated that while “questions of when life or personhood begin appear to be questions of fact susceptible of straightforward answers, we hold that the answers to such questions in fact are complex amalgams of factual and moral judgements” and acknowledged that “we do not want to see a situation in which human embryos are frivolously or unnecessarily used in research but we are bound to take account of the fact that the advances in the treatment of infertility, which we have discussed in the earlier part of this report, could not have taken place without such research; and that continued research is essential, if advances in treatment and medical knowledge are to continue”
These considerations led to a discussion of what is an embryo and, importantly, when does ‘an individual’ emerge during development. This was not a question of what Life is or when it begins, after all cells are living systems, but rather of whether it was possible to identify a moment in development at which a human being emerges that could guide the establishment of limits for embryo research (2). The Warnock committee solicited and considered views from over 300 experts working in the field of human reproduction as well as from members of the public in the form of 695 letters and also took into consideration religious views, which are diverse – for most Catholics the human being is traced back to the moment of conception whereas for Muslims and Jews it emerges on day 40 from fertilization (3-6). Scientific and medical views also varied; some saw that the moment to stop research should be when the blastocyst implants, around day 5 or 6 after fertilization, while others were of the view that the distinguishing feature of an individual is ‘sentience’ and that therefore, the moment should be linked to the appearance of the brain; around days 22 or 23 according to the embryos in the Carnegie collection. In the end, it was agreed “that no live human embryo derived from in vitro fertilisation, whether frozen or unfrozen, may be kept alive, if not transferred to a woman, beyond fourteen days after fertilisation, nor may it be used as a research subject beyond fourteen days after fertilisation”
The date, though a compromise of several views (1, 7), was not a choice of an arbitrary embryological middle ground between implantation and the emergence of the brain primordium, and owes much to the views of developmental biologist Anne Mclaren, a member of the committee. As she put it “If I had to point to a stage and say ‘This is when I began being me’ I would think it would have to be here” (3). Day 14 represents a significant change in the fortunes of the zygote. Before this time, but not after, any splitting of the cellular mass that results from fertilization will give rise to two complete individuals -identical twins arise in this manner- and importantly it is at this time that the cells begin the process of gastrulation which the committee agreed, but not unanimously (2, 7), was associated with the emergence of the individual. Gastrulation, a self-organized multicellular choreography that applies to all Metazoa, generates the germ layers and positions them with reference to an orthogonal coordinate system that will orchestrate the emergence of tissues and organs (8). In mammals, as in most amniotes, the distinguishing feature of this process is the so called ‘primitive streak’, a directional furrow carved by the cells in the epiblast -the name for the embryo just before gastrulation- that defines the anteroposterior axis of the organism thus demarcating the outline of the individual. The choice was not without controversy but accounts for some relevant biological facts e.g. the emergence of the unique axial organization of the organism, and the emergence of the brain as a consequence of gastrulation. In 1984 we did not know much about mammalian embryos but much has happened since, in particular the ability to grow fertilized eggs in the dish has been extended (9-11) opening up a discussion for a possible re-evaluation of a rule that has served as the central reference for thought and discussion of human embryology for over thirty years (3, 12, 13). However, for the moment, the guidelines have not changed and, importantly, technical challenges remain as under the best current culture conditions few embryos grown in culture make it to day 14 and even those that do, reach this landmark in poor physiological condition.
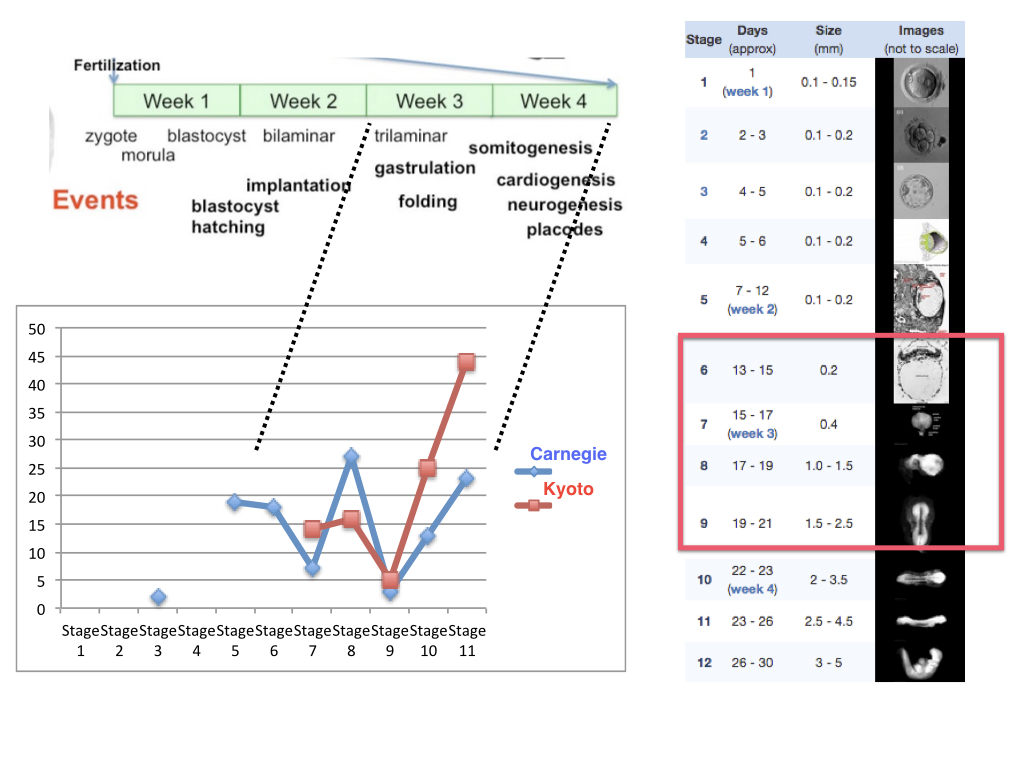
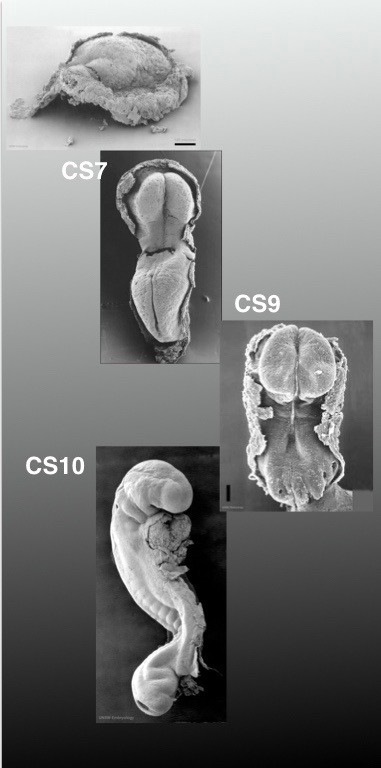
While progress is made in the regulatory and technical issues associated with the 14-day rule, we want and need to learn more about human embryos and, in particular about the ‘black box’ of human development, a notion often used with reference to two stages: implantation and gastrulation. The term refers to the fact that we know very little about these stages largely because, notwithstanding ethical constraints, the embryos are not easily accessible. In the case of implantation, the process is within the 14-day rule and it is very likely that over the coming years improved culture methods will create possibilities to explore these stages in detail by increasing the proportion and health of embryos that develop into these stages. Gastrulation is a different story. For the moment it is out of legal bounds and existing culture conditions are unlikely to produce healthy embryos in culture soon. Furthermore, solving these technical issues will require first solving the challenges associated with the implantation period and therefore, progress is likely to be slow. Furthermore, this work should be carefully monitored to avoid stunts and headlines about one-off events that will harm long term research progress by losing public and governmental support because of ill-founded hype. At these very early stages, even the mere observation of embryos is complicated because such embryos, which in theory can be obtained ethically from consensual or spontaneous abortions, are very difficult to find as can be seen in their underrepresentation in the Carnegie and Kyoto collections (see Figure which shows the number of embryos at early stages in the Kyoto and Carnegie collections). Altogether, these circumstances make this crucial time in human development inaccessible to study and certainly to experimentation. Non-human primates could, in theory, be an alternative for these studies but, sensibly, their embryos are subject to most of the same rules and regulations as human embryos. Therefore, if we want to gain some insights into human gastrulation, we need an alternative.
One solution to this impasse is provided by Embryonic stem cells (ESCs), clonal derivatives from early mammalian embryos that can be differentiated into any cell type of an organism and maintain this potency over many generations in culture. They were discovered in the 1980s in mice (14) and since have provided an immensely powerful tool for the study of mammalian development. They have also made the mouse a rightly crucial reference for the study of human biology, including the early stages of development. However, there are obvious and clear differences between rodents and primates with regard to the structure of the blastocyst, its implantation into the uterus and the process of gastrulation, which in humans takes place in a disc-like cellular ensemble which in the mouse takes the shape of a cylinder (15-17). Importantly, the time scale of the events is drastically different: implantation and gastrulation take about one and a half days each in the mouse, whereas in humans, it is about five or six days each. The effects of these differences on the cellular events that shape early development is not known but there is increasing evidence that there are also molecular differences between rodents and primates (18-20) that require the development of human developmental biology.
In 1998, ESCs were derived from human blastocysts by James Thomson (21). The work was privately funded because there was a ban on any work associated with human embryos. Shortly afterwards, in 2001, the US government imposed a ban on Federal funding of human ESCs (hESCs) on the basis of ethical and religious reasons: in order to get hESCs, blastocysts have to be destroyed and for those who believe that at this stage there is not only an embryo but also an individual, this process of ESC derivation implies the destruction of human life. The discussion harks back to some of the issues of the Warnock committee, specifically to the definition of an individual in the context of an embryo. Arguments were put forward that embryos used for hESC derivation were, always, surplus from IVF and that no embryos were created for the express purpose of deriving ESCs but this did not convince individuals with firm principles; it still does not. As it often happens, in the end a compromise was found on the balance between risk (destruction of human life) and benefit (the potential to address human disease) and a number of hESC lines were derived under defined ethical conditions that would be, and are, used by researchers as a reference (22). The efforts in the USA were followed by similar initiatives in the rest of the world and today there are a number of stem cell banks that regulate the distribution of these cell lines for research purposes. Importantly in 2008 it was shown that it is possible to generate hESC lines without destroying an embryo (23), which eased some of the ethical concerns. However, it was the discovery of induced Pluripotent Stem Cells (iPSCs) that changed the landscape and removed any ethical concerns to the use of hESCs for experimental purposes. In 2006 research led by Shinya Yamanaka showed that a cocktail of four transcription factors could transform any adult differentiated cell into a cell with the properties of an ESC (24, 25). What this meant was that one does not need to use embryos to obtain ESCs. iPSCs are not completely identical to ESCs but for most practical purposes they are and, ongoing research will polish the existing differences. Importantly, the all-important feature of any pluripotent stem cell, but specially of iPSCs, of being able to give rise to most, if not all, cell types of an organism, opened up the possibility of studying this process at any stage of development without, in principle, having to address the considerations implicit in the Warnock report.
However, while cells in dish are a good system to study cell growth and diversification, and has yielded some useful results with therapeutic potential, they are not the organized whole of tissues and organs whose ensemble is the hallmark of an organism or an embryo. The discovery of the ability of ESCs to self-organize under chemical steerage, into structures reminiscent of specific organs, organoids (26, 27), opened up a prelude for a new regenerative medicine with a developmental biology basis (28, 29). Much of this work was inspired by results with mouse ESCs but it is in the context of hESCs and, in particular iPSCs, where it has hinted at its huge potential. From a strict point view of developmental biology this work has revealed an unexpected modularity to mammalian development. If you stop and think about it, there is a surprise in the disembodied emergence of eye cups, regions of the brain, intestines, livers, pancreas but it is this autonomy and the use of iPSCs which mean that there is no need to think about embryos, though there are recommendations i.e. this work is not without ethical oversight. Beyond organoids, research with mouse ESCs has established conditions under which they can be coaxed to form a series of structures that resemble various stages of early development, including blastocysts and even reproduce the outcome of gastrulation (30). This menagerie serves as a collection of models of early development that expand our experimental repertoire. Because they can be compared with embryos, they provide a useful tool for experiments which are difficult, if not impossible, with embryos. These observations have opened up the door to the development of similar structures from hPSCs (ESC and iPSC) which, on the basis of what we have learnt from mouse, can provide insights into the ‘black box’ of human development without the need to use embryos. While these structures create a bridge between the 14-day rule and the embryo, thus providing a solution to some of the issues raised by the Warnock committee, they raise questions of their own (31). These issues and, in particular the science associated with these structures, will be explored in the final section of this series.
References
1. Warnock, Mary. 1984. Report of the Committee of Inquiry into Human Fertilisation and Embryology. London: Her Majesty’s Stationery Office.
2. Warnock, M. (1985) The Warnock report British Med. J. 291, 187-189.
3. Cavaliere, G. (2017) A 14-day limit for bioethics: the debate over human embryo research. BMC Medical Ethics 18:38.
4. (2017) Catholic view Nuffield Council on Bioethics; human embryo culture
5. Suleman, M. (2017) Islamic perspectives in the moral and policy significance of developmental threshold. In “Nuffield Council on Bioethics; human embryo culture” pp 61-64.
6. Shenker, J. (2008) The beginning of human life. Status of the embryo; perspectives in Halakha. J. Assist Reprod. Genet. 25, 271-276.
8. Solnica-Krezel, L. and Sepich, D. (2012) Gastrulation: making and shaping germ layers Ann Rev. Cell Dev, Biol. 28, 687-717.
9. Deglincerti, A. et al. (2016) Self-organization of the in vitro attached human embryo Nature 533, 251-254.
10. Shahbazi, M. et al. (2016) Self-organization of the human embryo in the absence of maternal tissues Nature Cell Biol 18, 700-708.
11. . Xiang, L. et al. (2019) A developmental landscape of 3D cultured human pregastrulation embryos. Nature 577, 537-542.
12. Hyun, I., Wilkerson, A. and Johnston, J. Embryology policy: Revisit the 14-day rule. Nature 533, 169-171.
13. Nuffield Council on Bioethics; human embryo culture (2017).
14. Evans, M. (2011) Discovering pluripotency: thirty years of mouse embryonic stem cells. Nature Rev Mol. Cell Biol. 12, 680686.
15. Rossant, J. and Tam, P. (2017) New Insights into Early Human Development: Lessons for Stem Cell Derivation and Differentiation. Cell Stem Cell 20, 18-28.
16. Boroviak, T. and Nichols, J (2017). Primate embryogenesis predicts the hallmarks of human naive pluripotency. Development 144, 175-186.
17. NB It is remarkable that there is little, if anything, by way of literature on human gastrulation. What little there is refers to discussions of the Carnegie collection on line https://www.ehd.org/virtual-human-embryo/ and to a Youtube video which aims to summarize what is known from these images https://www.youtube.com/watch?v=yO0BEGCusHs
18. Barry, C. et al. (2017) Species specific developmental timing is maintained by pluripotent stem cells ex utero. Dev Biol. 423, 101-110.
19. Matsuda, M. et al. (2020) Recapitulating. The human segmentation clock with pluripotent stem cells. Nature 580, 124-129.
20. Rayon, T. e al. (2020) Species-specific developmental timing is associated with global differences in protein stability in mouse and human. https://www.biorxiv.org/content/10.1101/2019.12.29.889543v1
21. Thomson, J. A. et al. (1998) Embryonic stem cell lines derived from human blastocysts.Science 282, 1145–1147.
22. Cowan, C. et al. Derivation of Embryonic stem cell lines from human blastocysts N Engl J Med 2004 Mar 25;350(13):1353-6.
23. Chung, Y. et al. (2008) Human embryonic stem cell lines generated without embryo destruction. Cell Stem Cell 2, 113-118.
24 Takahashi, K. and Yamanaka, S. (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663-676.
25 Takahashi, K. et al. (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861-872.
26. Sato, T. et al. (2009) Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche.Nature 459, 262-265.
27 Eiraku, M. et al. (2011) Self-organizing optic-cup morphogenesis in three-dimensional culture Nature 472, 51-56.
28 Clevers, H. (2016) Modelling development and disease with organoids. Cell 1586-1597.
29 Sasai, Y. (2013) Next-generation regenerative medicine: organogenesis from stem cells in 3D culture. Cell Stem Cell 12, 520-530.
30. Shahbazi, M., Siggia, E. and Zernicka-Goetz, M. (2019) Self-organization of stem cells into embryos:a window on early mammalian development. Science 364, 948-951.
31. Rivron, N. et al. (2018) Debate ethics of embryo models from stem cells. Nature 564, 183-185.