III. Models of human development
Pluripotent stem cells (PSCs: ESCs and iPSCs) create opportunities to address the ethical and technical constraints associated with the use of human embryos. In order to discuss and evaluate these opportunities it is important first to provide a context for this work and look at its roots and assess its limitations.
These days one has the impression that Embryonic Stem Cells (ESCs) are collections of genes, of epigenetic modifications all revealed by barcodes and sophisticated genomic technologies but, actually they are cells and their surname is embryonic, and cells are the units of the embryo, the bricks, tiles and stones with which tissues and organs build themselves. For all that these days we relish in the description of living systems at the level of single cell transcriptomics, the last thing these studies deal with is cells and, let’s face it, genes are mere instructions to make cells, more cells, different cells. Our emphasis on genes is likely to be derived from the fact that, by and large, we understand how they work, which allows us to manipulate them with some precision e.g gene cloning, CRISPR, PCR, synthetic circuits, etc. Over the last thirty years, genes have provided us with a narrative of development reflected in a link between their organization into Gene Regulatory Networks (GRNs) and specific processes e.g segmentation in vertebrates and invertebrates, the patterning of the nervous system, of the wing of a fly or of the pharynx of a nematode. So called model organisms have been at the centre of this endeavour in the form of genetic screens: you want to know how a neuron is made? Start mutating genes and assess which ones are needed for this job and then see how these relate to each other. The result: a GRN associated with that neuron. It does not tell us how that neuron comes to be or how it relates to other neurons, but it tells us that those genes are needed for that neuron to be. At the end of this exercise we still lack an understanding of the relationship between genes and cells, between genotype and phenotype.
In a tradition that precedes the systematic application of genetic analysis to the study of development, using organisms not suited to genetic analysis, like amphibia and birds, Experimental embryology provided a different perspective, one based on the behaviour of cell ensembles that gave us the Spemann-Mangold organizer, the elements that pattern the vertebrate limb and even hints of gradients in insects 1. These studies, which are ‘gene free’ have provided insights about development that have withstood well the passage of time. Furthermore, they already revealed the self-organising potential of the pluripotent cells of early embryos and thus presaged the current organoid field 2.
In an ideal world, one would like to combine the two traditions: be able to interrogate cell ensembles and use Genetics not only to see what a specific gene is required for but, more importantly, as a tool to perturb the system. For example, given our knowledge of the molecular machines mediating chromatin remodelling or cytoskeletal dynamics, we can use mutations in specific elements of those machines to test the role of these activities in the building of those structures.
Mammalian embryos have always been challenging experimental subjects from both perspectives and therefore are not good candidates for the ideal system. Their size and reproductive cycles make most of them unsuitable for genetic analysis and their development in the maternal womb, nearly impossible subjects for Experimental embryology. Nonetheless, throughout the XX century, the mouse has become a reference for mammalian developmental biology, mostly from a genetic point of view 3, and has provided a number of insights about the early stages of mammalian embryos; in particular about the emergence of embryonic and extraembryonic lineages and the machinery of gastrulation 4,5. In this realm, and as discussed in the last post, human embryos remain off bounds because of ethical and technical reasons. On the basis of genetic conservation between groups and some obvious similarities at the level of embryonic organization, the mouse has become a reference for the human embryo. However, even at the early stages there are differences between the two systems 6 and this situation is not satisfactory; enter PSCs.
In Science, sometimes, an opportunity arises in a paradox, in a contradiction and ESCs present us with one that is already evident in the mouse. In adherent culture, PSCs can be steered to differentiate into any cell type of the organism under the guidance of signalling molecules, but they rarely form coherent structures that echo the organization of the embryo. However, if the same cells are placed into a preimplantation embryo they will integrate with the cells of the host and participate in normal development. What cues does the embryo have that instil the organization of those cells?
In adherent culture, the differentiation of PSCs, whether mouse or human, is heterogeneous and heterochronic. At the end of an experiment what is left on the plate is a mixture of cell types at different stages of development; not a great model for the exquisite organization of an embryo. Because we can select and amplify what we want, these experiments can create a mirage of success. The situation is not much better in Embryoid bodies (EBs) large cell aggregates that when induced to differentiate exhibit a similar level of disorder as the adherent culture but this time in 3D 7. However mixed experimental protocols and some attention to the culture conditions turn the EBs into organoids thus revealing a remarkable organ building potential in the PSCs. Much to think about in these alchemic experiments that deliver sometimes spectacular results but they raise many questions which are too often ignored 8.
In 2014, building on work from the group of Peter Zandstra on the reproducibility of ESC-based culture systems 9, Aryeh Warmflash, working with Ali Brivanlou and Eric Siggia, showed that hESCs plated on micropatterned substrates of defined sizes, and challenged with BMP4, the signal that initiates the patterning of the mammalian embryo, would organize gene expression into radially concentric patterns that mimicked the organization of germ layers in the mammalian embryo 10. It was an abstracted version of the early embryo, but it had too many similarities to be ignored. Despite the clear patterning of the cell ensemble, the patterning did not outline a body plan and raised the question of what would happen if the experiment could be translated to three dimensions. The emergence of organoids boded well for the translation from two to three dimensions; a process that, as much in this field, owed a great deal to work with mouse systems.
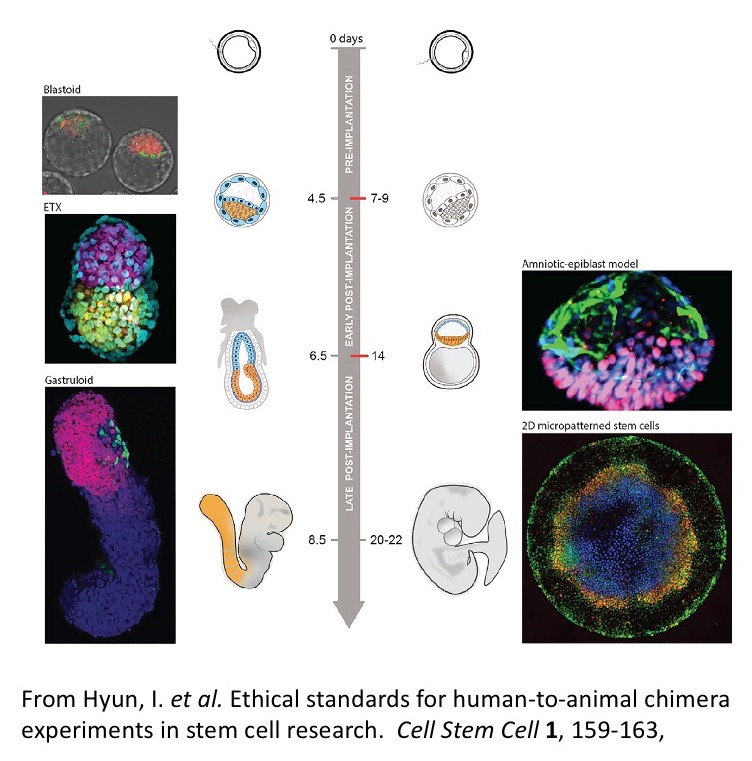
The emergence of the body plan is deeply associated with the process of gastrulation, a multicellular choreography that shapes the embryo out of the ball of cells that results from the proliferation of the zygote and that, as we saw last week, also represents the ethical line for in vitro culture of human embryos. The hallmark of the onset of gastrulation is the expression of the T box transcription factor Brachyury (Bra) at one end of the embryonic cell ensemble that results from proliferation after fertilization. The pattern of Bra expression, which is universal, is a sign that the mass of cells has acquired a polarity and triggers a directional cascade of Epithelial Mesenchymal Transitions (EMTs) that signals the emergence of an individual organism through the definition of its anteroposterior axis. The micropatterned arrangements of hESCs lack this axial organization though they exhibit expression of BRA and, as they would in the embryo, BRA expressing cells execute an EMT (see also 11). This observation led to the suggestion that these structures are, somehow, rehearsing some features of gastrulation 12-14 and, although it is difficult to see how these concentric domains of gene expression relate to an embryo, perhaps we need to revise our notions of what the process of gastrulation is (more on this here soon). Regardless of these abstract considerations, the fact is that an embryo is a three dimensional structure that, in the case of a mammal, emerges encased within a niche of extraembryonic tissues which also endow it with its initial coordinate system. Therefore it is possible that in order to recapitulate the events that transform the starting cellular mass, represented by PSCs, into the outline of an embryo in vitro, we need to follow the steps that take place in vivo and tinker with the full set of components: embryonic and extraembryonic cells. On this hunch, over the last few years working with mouse ESCs, the laboratory of Magdalena Zernicka-Goetz has revealed that, when placed together with stem cells for extraembryonic lineages (Trophoblast, TS and primitive endoderm, XEN, cells), they will give rise to structures that resemble the pregastrulation embryo 15,16. Furthermore when these cells are placed together under defined and precise conditions, as in the work of Nicolas Rivron, the frequency of these events is high and the cells form remarkable copies of the preimplantation embryo, called blastoids 17. In some instances, these structures attempt the process of gastrulation 16 but, despite the presence of a focus of Bra expression in some of the structures, they fail. Similar work with human ESCs and TS, leads to entities with an axis that resembles the AP axis of the embryo 18 and a step forward was given when the laboratory of Jianping Fu reported the emergence of structures that resembled the pregastrulation human embryo from single human PSCs 19,20. These structures make it to the onset of gastrulation when, as in the case of the mouse PSC-derived structures, the system collapses. Thus, an intriguing common denominator of all these studies is that while they reproduce the early embryonic structures, they do not progress through gastrulation.
A few comments before moving on. First, notice that in all this work — as well as that of organoids — the resulting structures result from the manipulation of cells, not of genes. What we tinker with is cells to induce Cell Regulatory Networks (CRNs) that, when made reproducible, allow us to build in order to understand. The structures also raise many questions for, in these systems, it is from the differences with the embryo that we learn. For example, to date although some of these structures exhibit an uncanny resemblance to conceptuses (technical name for the ensemble of extraembryonic and embryonic tissues before implantation) and will be in principle accepted by the uterus, they do not develop beyond pregastrulation stages raising the question of what is missing in the engineered structures. Does the uterine environment provide signals that endow the conceptus with physiological functionality in a manner that is not replicated in vitro? Importantly, none of these structures undergo gastrulation, in the sense of yielding a body plan. In the case of the human, the 14-day rule appeared to become a barrier that cells refuse to cross.
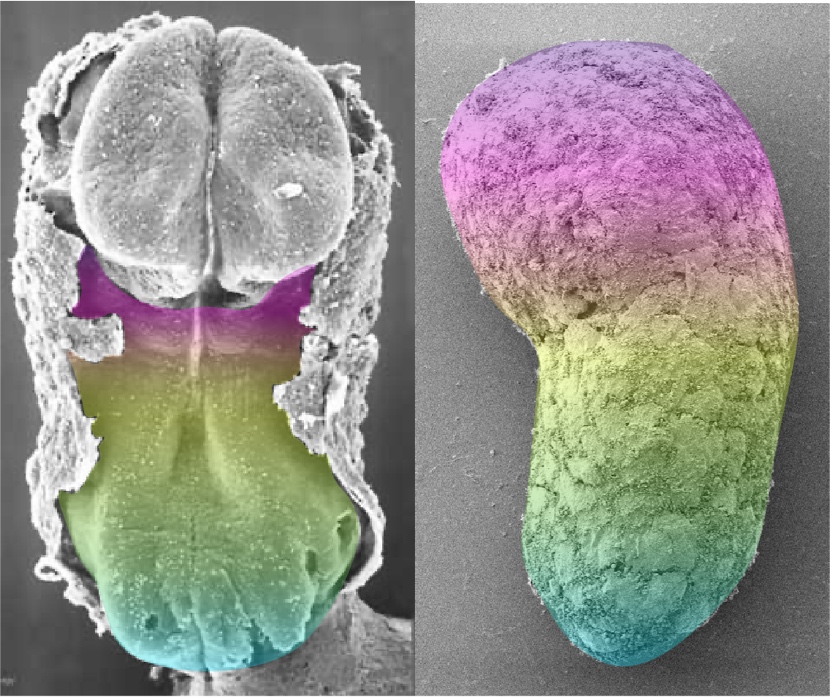
As the micropatterns were being used to ask questions about early cell fate specification, reports emerged that under defined culture conditions mouse ESCs could be directed to form structures that, after five days, exhibited a genetic blueprint remarkably similar to that of the vertebrate body plan. The work was inspired by Yusuke Marikawa’s experiments with aggregates of Embryonal Carcinoma P19 cells 21; an important manuscript that was largely ignored due, most likely, to the lack of hype by the authors and the publication in what would be deemed an archival, specialist journal. Extension of these observations to ESCs through an adaptation of protocols for adherent culture differentiation to three dimensional (3D) aggregates, yielded what further studies have shown to be an outline of the mouse embryo, its body plan 22-24. These structures could be deemed a 3D version of the micropatterns, though with important differences which make them closer to embryos. In particular, gastruloids mirror the temporal events of the embryo, their complexity evolves over time and they acquire and use a coordinate system to place the different tissue primordia relative to each other. As in the embryo this organization is the outcome of the gastrulation process, it is for this reason that these structures have been called ‘Gastruloids’ 23. There is much that is surprising about these structures but perhaps the most intriguing one is that while they seem to have disentangled a genetic blueprint from morphogenesis: the patterns of gene expression are spatially organized but the different gene expression domains lack morphogenesis. Recent work has begun to untangle and address the reasons for this dissociation. They also lack a brain and extraembryonic tissues. Gastruloids bear the possibility of bringing together Genetics and experimental embryology in a manner that might not have been expected in mammals and there are promising results already in this direction 25-28. Recently, gastruloids have been obtained from human ESCs 29. As for their mouse counterparts, human Gastruloids lack a brain primordium and extraembryonic tissues and thus, from the point of view of the discussions of the Warnock committee, lack full embryonic potential. This means that, in principle, as many of the other PSC-based structures, they should not be a cause of ethical concerns 30. Importantly, and as their mouse counterparts, they seem to undergo a gastrulation-like process that yields the outline vertebrate body plan. Thus, we now have a collection of cell based models to study the early stages of human development.
The suite of structures that are being obtained from PSCs represent a collection of models of mammalian embryonic development. A model is a representation of a reality, in this case the embryo, that contains some features of that reality and ignores others. A model is, in some ways, a simulation of that reality and, as long as we make sure that its component elements reflect the reality, it will help us understand its workings; a comparison with the reality it represents is a key aspect of the process. The aim of a model is not to copy the reality it represents but to understand it and for this, we need to look and see the opportunities that such constructs offer. This is clear in the models of embryonic development mentioned above which have revealed a number of unsuspected features, in particular the roles that geometry and mechanics play in organizing cell ensembles or a surprising modularity and self-assembly/self-organising properties of PSCs. However, as already stated, the value of a model depends in how much it reflects the reality it represents. In the case of the mouse models it is easy to answer this question for direct comparisons can be made between the in vitro structures and the embryos. These have been made and have revealed parallels and differences that allow us to assess whether those differences teach us something or are artefacts of the experimental system; though even here we can learn. The human models are extremely important, as they provide a bridge across the 14-day rule for embryonic experiments and through it, a unique window into a most important process that is not easily accessible to experimentation. However, it is that inaccessibility that presents the challenge to know how much the model reflects the reality it aims to represent. In the case of the pregastrulation embryo it is possible to compare with the embryo 19 but in the case of gastruloids for the moment this is not possible. This might change as the recommendations associated with the 14-day rule pertain to the in vitro culture of embryos with full individual potential but not the ability to recover embryos. Thus, in principle, it should be possible to obtain embryos from miscarriages and abortions with ethical and legal oversight, and these could be used as reference for analysis. In any case for now, and as it has been the case over the last thirty years, the mouse remains a reference and the triangulation mouse embryo – mouse Gastruloid – human Gastruloid is a good start to understand the early patterning events of the human embryo
The menagerie of ESC-derived embryo-like entities that is emerging (and very likely will continue to emerge) are an intriguing complement to the organoid field and represent a toolbox to address the issues that we posed in the first post:
- To learn how and why we are different from other organisms;
- To investigate the origin and causes of many pathologies and with this understanding explore ways to relieve them;
- To understand, and perhaps ameliorate, the causes of early miscarriages.
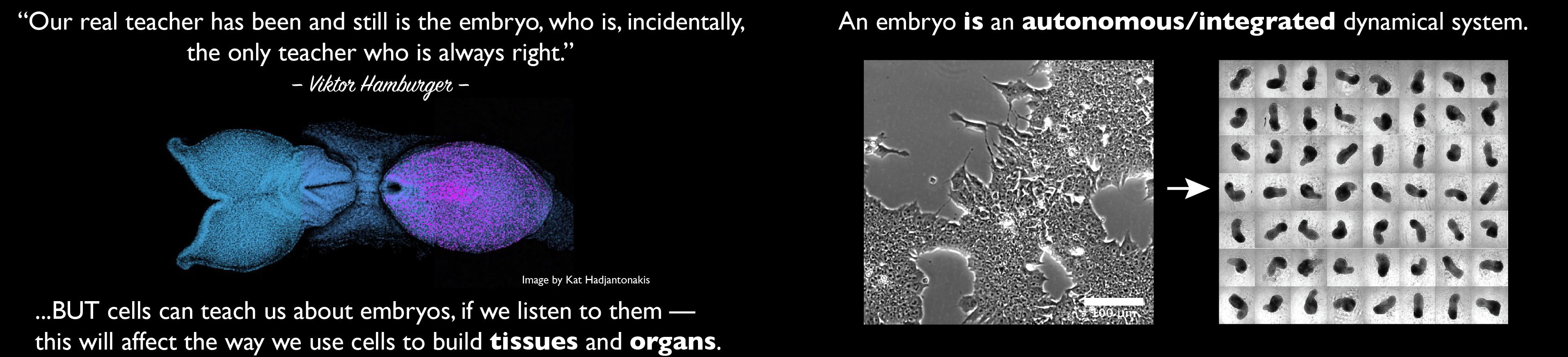
This will be done by a powerful combination of classical experimental embryology and modern genetic engineering that these entities allow 31. In the end, these models highlight how far we have come since the 1980s in understanding mammalian embryos and encourage and support a need for a reassessment of those discussions. At a more fundamental level, these structures challenge our notion of what is an embryo, of the process of gastrulation, of how do we come to be. It is likely that in the answer to these questions lies new vistas of Developmental Biology and, maybe, of its applications to Medicine but for this to happen we need to be critical of our work and set up the same standards as the embryo. In an often quoted statement, the famous embryologist Viktor Hamburger, custodian of Experimental embryology 1, said that ‘our real teacher has been, and still is, the embryo who is, incidentally the only teacher who is always right’. There is much truth in those words but, if we want to learn about its wisdom we need to go, as always, to the sources and these are the cells. Cells, in the way that they are being used in the work summarized here, can teach us much about how embryos build themselves, if we listen to them.
NB. This is a blog post not a review, thus by necessity the references are not exhaustive but aim to be examples of the issues that are raised.
References
- Hamburger, V. The heritage of experimental embryology. (Oxford University Press, 1988).
- Hurtado, C. & De Robertis, E. M. Neural induction in the absence of organizer in salamanders is mediated by MAPK. Dev Biol307, 282-289, doi:10.1016/j.ydbio.2007.04.049 (2007).
- Artzt, K. Mammalian developmental genetics in the twentieth century. Genetics 192, 1151-1163, doi:10.1534/genetics.112.146191 (2012).
- Arnold, S. J. & Robertson, E. J. Making a commitment: cell lineage allocation and axis patterning in the early mouse embryo. Nat Rev Mol Cell Biol 10, 91-103, doi:10.1038/nrm2618 (2009).
- Rossant, J. & Tam, P. P. Emerging asymmetry and embryonic patterning in early mouse development. Dev Cell 7, 155-164, doi:10.1016/j.devcel.2004.07.012 (2004).
- Rossant, J. & Tam, P. P. L. Exploring early human embryo development. Science 360, 1075-1076, doi:10.1126/science.aas9302 (2018).
- Brickman, J. M. & Serup, P. Properties of embryoid bodies. Wiley Interdiscip Rev Dev Biol 6, doi:10.1002/wdev.259 (2017).
- Huch, M., Knoblich, J. A., Lutolf, M. P. & Martinez-Arias, A. The hope and the hype of organoid research. Development 144, 938-941, doi:10.1242/dev.150201 (2017).
- Bauwens, C. L. et al. Control of human embryonic stem cell colony and aggregate size heterogeneity influences differentiation trajectories. Stem Cells 26, 2300-2310, doi:10.1634/stemcells.2008-0183 (2008).
- Warmflash, A., Sorre, B., Etoc, F., Siggia, E. D. & Brivanlou, A. H. A method to recapitulate early embryonic spatial patterning in human embryonic stem cells. Nat Methods 11, 847-854, doi:10.1038/nmeth.3016 (2014).
- Turner, D. A., Rue, P., Mackenzie, J. P., Davies, E. & Martinez Arias, A. Brachyury cooperates with Wnt/?-Catenin signalling to elicit Primitive Streak like behaviour in differentiating mouse ES cells. BMC Biol 12, 63, doi:10.1186/s12915-014-0063-7 (2014).
- Heemskerk, I. & Warmflash, A. Pluripotent stem cells as a model for embryonic patterning: From signaling dynamics to spatial organization in a dish. Dev Dyn245, 976-990, doi:10.1002/dvdy.24432 (2016).
- Martyn, I., Siggia, E. D. & Brivanlou, A. H. Mapping cell migrations and fates in a gastruloid model to the human primitive streak. Development 146, doi:10.1242/dev.179564 (2019).
- Massey, J. et al. Synergy with TGFbeta ligands switches WNT pathway dynamics from transient to sustained during human pluripotent cell differentiation. Proc Natl Acad Sci U S A 116, 4989-4998, doi:10.1073/pnas.1815363116 (2019).
- Harrison, S. E., Sozen, B., Christodoulou, N., Kyprianou, C. & Zernicka-Goetz, M. Assembly of embryonic and extraembryonic stem cells to mimic embryogenesis in vitro. Science 356, doi:10.1126/science.aal1810 (2017).
- Sozen, B. et al. Self-assembly of embryonic and two extra-embryonic stem cell types into gastrulating embryo-like structures. Nat Cell Biol20, 979-989, doi:10.1038/s41556-018-0147-7 (2018).
- Rivron, N. C. et al. Blastocyst-like structures generated solely from stem cells. Nature 557, 106-111, doi:10.1038/s41586-018-0051-0 (2018).
- Simunovic, M. et al. A 3D model of a human epiblast reveals BMP4-driven symmetry breaking. Nat Cell Biol 21, 900-910, doi:10.1038/s41556-019-0349-7 (2019).
- Zheng, Y. et al. Controlled modelling of human epiblast and amnion development using stem cells. Nature 573, 421-425, doi:10.1038/s41586-019-1535-2 (2019).
- Shao, Y. et al. A pluripotent stem cell-based model for post-implantation human amniotic sac development. Nat Commun 8, 208, doi:10.1038/s41467-017-00236-w (2017).
- Marikawa, Y., Tamashiro, D. A., Fujita, T. C. & Alarcon, V. B. Aggregated P19 mouse embryonal carcinoma cells as a simple in vitro model to study the molecular regulations of mesoderm formation and axial elongation morphogenesis. Genesis 47, 93-106, doi:10.1002/dvg.20473 (2009).
- Beccari, L. et al. Multi-axial self-organization properties of mouse embryonic stem cells into gastruloids. Nature 562, 272-276, doi:10.1038/s41586-018-0578-0 (2018).
- van den Brink, S. C. et al. Symmetry breaking, germ layer specification and axial organisation in aggregates of mouse embryonic stem cells. Development 141, 4231-4242, doi:10.1242/dev.113001 (2014).
- Turner, D. A. et al. Wnt/beta-catenin and FGF signalling direct the specification and maintenance of a neuromesodermal axial progenitor in ensembles of mouse embryonic stem cells. Development 141, 4243-4253, doi:10.1242/dev.112979 (2014).
- Veenvliet, J. et al. Mouse embryonic stem cells self-organize into trunk-like structures with neural tube and somites. bioRxiv doi: https://doi.org/10.1101/2020.03.04.974949 (2020).
- Noemi, M. et al. Early neurulation recapitulated in assemblies of embryonic and extraembryonic cells. bioRxiv doi: https://doi.org/10.1101/2020.02.13.947655 (2019).
- Cermola, F. et al. Gastruloid development competence discriminates different states of pluripotency between naïve and primed. bioRxiv doi: https://doi.org/10.1101/664920 (2019).
- Turner, D. A. et al. Anteroposterior polarity and elongation in the absence of extra-embryonic tissues and of spatially localised signalling in gastruloids: mammalian embryonic organoids. Development 144, 3894-3906, doi:10.1242/dev.150391 (2017).
- Moris, N. et al. An in vitro model of early anteroposterior organization during human development. Nature582, 410-415, doi:10.1038/s41586-020-2383-9 (2020).
- Hyun, I. et al. Ethical standards for human-to-animal chimera experiments in stem cell research. Cell Stem Cell 1, 159-163, doi:10.1016/j.stem.2007.07.015 (2007).
- Moris, N., Martinez Arias, A. & Steventon, B. Experimental embryology of gastrulation: pluripotent stem cells as a new model system. Curr Opin Genet Dev 64, 78-83, doi:10.1016/j.gde.2020.05.031 (2020).